The Anatomy of a Diamond: Physical, Chemical, Optical Properties
A diamond is a mineral composed of pure carbon, and notable for its isometric crystal structure. Each carbon atom is attached to four other carbon atoms. It’s the hardest mineral on earth. Given its structure, it has unique physical, chemical, and optical properties.
Book an appointment with our experts to learn more about lab-grown diamonds and how you can integrate them into your engagement ring or fine jewelry.
What’s the Anatomy of a Diamond?
As a three-dimensional object, a diamond has six main components: facets, table, crown, girdle, pavilion, and culet. While no two diamonds are identical, all diamonds share, to some extent, the same structural components.
Facet
Facets are flat surfaces on a diamond that are cut and arranged in specific geometrical patterns. The most common facets are step-cut facets, which are usually trapezoidal or rectangular, and brilliant facets, which are cut in triangular and kite-shaped facets. The way the facets are cut impacts the overall ability of a diamond to reflect and bounce light. Brilliance depends not only on the specific characteristics of one particular diamond, but also on the ability of a master-cutter to facet it.
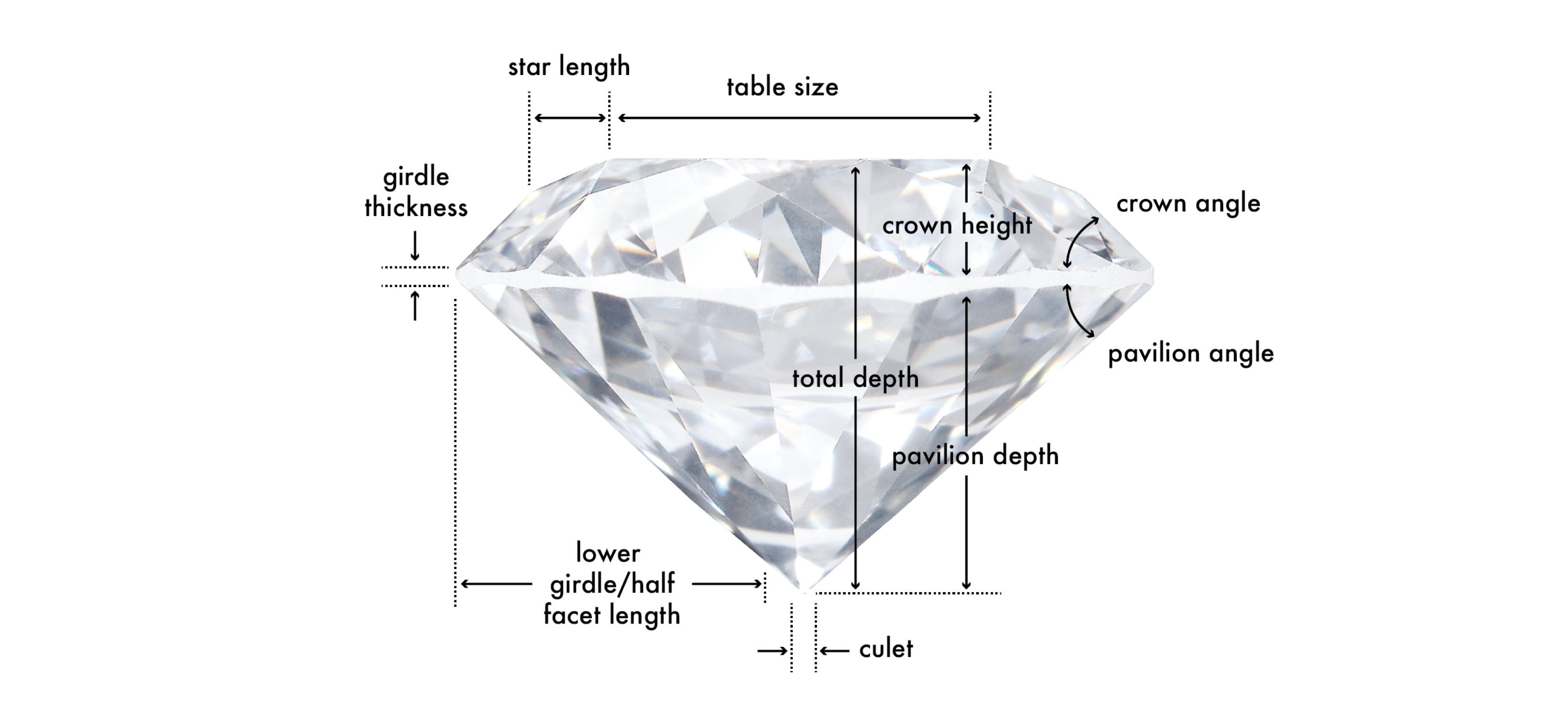
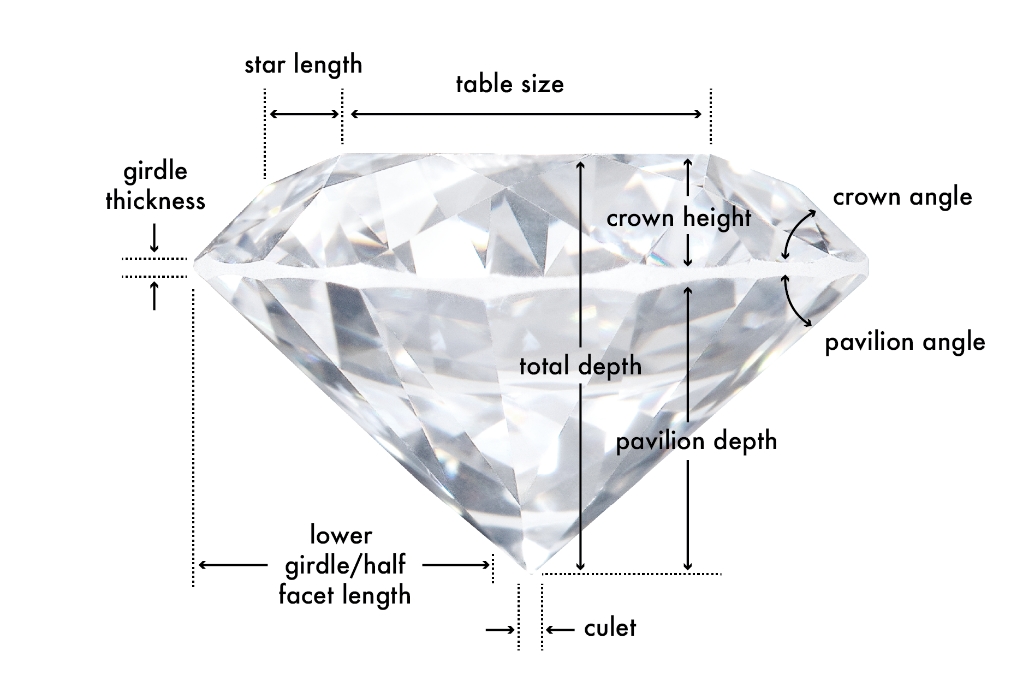
Table
In terms of anatomy, the Table is the largest polished facet located on the top of the diamond. The average table size is expressed in terms of percentage of the girdle’s diameter. In terms of round cut diamonds, a table that’s 52% to 62% of the girdle’s diameter contributes to an “Excellent” cut rating.
Crown
The crown is the top part of the diamond, located between the table and the girdle, and comprises several types of facets. The crown height is expressed as a percentage of the average girdle diameter, and it can affect both the dispersion and brightness of a diamond. An important characteristic of the crown is its angle. The crown angle in a well-cut diamond will be within 31.5 to 36.5 degrees. The crown angle directly contributes to the face-up appearance of a round brilliant cut diamond
Girdle
The girdle is the middle portion of a diamond and its widest edge, separating the crown from the pavilion. The girdle thickness is something you want to be mindful of. A thick girdle is less desirable because it adds weight in a place where it really does not contribute to the appearance of the diamond. On the contrary, a thick girdle will make a diamond face up smaller. A girdle that is too thin will make the diamond more fragile.
Pavilion
The pavilion refers to the lower portion of the diamond, extending from the girdle to the culet (the pointed facet at the bottom of the diamond). Important characteristics of the pavilion are the pavilion depth and the pavilion angle. If the pavilion is too deep or too shallow it will either trap the light in the stone or let it escape from the sides. The pavilion angle impacts the diamond’s brightness and, in the case of a Round Brilliant, an angle between 40.6 and 41.8 degrees contributes to an “Excellent” cut rating. Not all diamond cuts have a pavilion. Rose cut diamonds are known for being dome-shaped and for having a flat bottom.

Be the first to know
Hear about our latest designs and upcoming events.
What are the Chemical Properties of a Diamond?
The diamond is the only mineral that is (almost) exclusively made of one single element. A diamond consists of at least 99.95% carbon atoms. The remaining 0.05% are trace elements that commonly influence the color of the diamond. In addition, the diamond has an isometric crystal structure: each carbon atom is surrounded by four other carbon atoms and they are all connected by covalent bonds. This creates a uniform, stable, and resistant structure that influences the diamond’s physical properties.
What are the Physical Properties of a Diamond?
The physical characteristics of a diamond are exceptional to the point that diamonds have many uses beyond jewelry and ornaments. Diamonds have the highest thermal conductivity and the highest sound velocity, and they also have high electrical resistance. A diamond is also inert: it means it does not react with the most corrosive substances. In addition, diamonds are the hardest known substance.
On the Mohs hardness scale, diamonds have a hardness of 10, which means that out of all minerals, they are they most resistant to being scratched. This does not mean that diamonds are indestructible though; diamonds are still prone to chipping and cracking from hard impacts.

Get your complimentary ring sizer!
Measure your ring size for the perfect fit right from the start.
What are the Optical Properties of a Diamond?
Optical properties indicate the way light interacts with a substance. The diamond's physical properties give the gemstone the highest possible type of luster of gemstones, which is the Adamantine luster. Luster refers to the light-reflecting characteristics of a mineral. Diamonds also have high dispersion: white light enters the gemstone and then scatters into rainbow-colored light.
Lab vs Mined Diamonds: Anatomy and Properties
Lab grown diamonds and mined diamonds share the same growth process, despite the fact that it occurs in different environments. This means that the resulting anatomy and properties are very similar—we don’t say identical because no two diamonds are exactly alike. In the case of mined diamonds, extreme heat and pressure 100 miles below the surface of the earth causes carbon atoms to create the crystal-like formations that are the main “building block” of a diamond.
The same thing happens in a laboratory setting, and VRAI and the Diamond Foundry share this process, but with a twist. “We started by studying the laws of nature. Diamonds are born from a fiery heat, so we set out to create a plasma of unprecedented energy density,” explain our scientists. After coding software to run tens of thousands of physics-based simulations, they built a warehouse with plasma reactors with hundreds of individually precision-engineered parts.
“Plasma is the fourth state of matter (the other states of matter being solids, liquids, and gasses in increasing energy levels). “In a plasma akin to a sun on earth, atoms can attach to nature's diamond crystal lattice and grow it in size,” the explanation continues. “One by one, the atoms stack on top of a thin foundation of diamond, extending the unique crystal structure of diamonds extracted from the Earth. Atom by atom, it grows into a pure, jewelry-grade diamond of gem size.”
Why are Lab-Grown Diamonds and Mined Diamonds Chemically identical?
Heat and pressure allow carbon to form the isometric crystal structure where each atom is bonded to four other atoms in a strong covalent bond. Heat and extreme pressure occur both under the surface of the earth and in a man-made foundry.
Why are Lab-Grown and Mined Diamonds Physically identical?
Once established that the growth process yields crystals with the same chemical properties, the physical properties of lab-grown diamonds and mine diamonds will mostly align too. Both lab-grown have the highest thermal conductivity and the highest sound velocity, and high electrical resistance. Neither lab nor mined diamonds react with the most corrosive substances and, on the MOHS scale, lab-grown diamonds and mined diamonds both have a 10/10 score.
Why are Lab-Grown Diamonds and Mined Diamonds Optically Identical?
Physical properties influence the optical properties of a diamond, and so it makes sense for both lab grown diamonds and mined diamonds to exhibit a similar range of refraction, dispersion and to have a similar luster. In addition, refraction and dispersion can be, to some extent, influenced by the proficiency of the master cutter, as the cutting and the faceting directly impacts the way a diamond looks and the way it scintillates.
Book an Appointment
Purchasing a diamond begins with educating yourself on a stone that has unique properties. When it comes to diamonds, the journey (education) is as rewarding as the destination (the purchase). Our experts will be able to guide you.